Give the Full Electron Configuration for Sulfur.
The sulfurS electron configuration will be 1s2 2s2 2p6 3s2 3p4. Table 1 mz Abundance 58 610 60 291 61 99 Give the symbol including mass number of the ion that would reach the detector first in the sample.

Sulfur Electron Configuration Youtube
It is defined as the arrangement of electrons of a molecule in a molecular orbital.

. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration is also mentioned in the table. Electron Configuration Chart of All Elements Full Chart January 1 2022 March 7 2021 by Admin. Here sulfur has four unpaired electrons.
Two p two fosters. The spectrum produced showed three peaks with abundances as set out in Table 1. Electron Configuration and Oxidation States of Sulfur.
The electron configuration of chlorine is illustrated below. Electron configuration of Sulfur is Ne 3s2 3p4. The sample was ionised by electron impact ionisation.
The superscripts represent the electrons present in each region of the periodic table. Sulfur S What element has a ion e- configuration of 1s2 2s2 2p6 3s2 3p6. Give the full electron configuration for sulfur.
_10Ne 1s22s22p6 Historical note. So the number of electrons 16. 1s2 2s2 2p6 3s2 3p6 4s1 3d5.
The electron configuration of sulfur excited state is S 16 1s 2 2s 2 2p 6 3s 2 3p x1 3p y1 3p z1 3d xy1. Ne3s23p1 Aluminium has atomic number 13 so the full electron configuration will be. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure.
What is a half-full electron configuration for Chromium Cr. Give the full electron configuration for sulfur. Electronic configuration.
See the answer See the answer done loading. Atomic number 16. 100 38 ratings 1s2.
The chemical symbol for Sulfur is S. In this case that is neon which is. Electron configuration chart of all Elements is mentioned in the table below.
The sum of these superscripts should equal the atomic number for a neutral atom. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. Sulfur is the SIXTH element in the THIRD row of the periodic tableSo it is the same as Neon but with a full 3s2 subshell and four electrons in its 3pWhen.
Chemistry questions and answers. Answer to Solved Give the full electron configuration for sulfur. Therefore it has 16 electrons in its outermost energy level.
Possible oxidation states are 46-2. View the full answer. The full electron configuration for each element.
The last electron is in the 4th period in the p region and the. Thus its electronic configuration is Ne3s 23p 5 or 1s 22s 22p 63s 23p 5. Give the full electron configuration for sulfur.
Chlorine has an electron configuration of 2-8-7. Sulfur needs another two electrons to have a stable arrangement. However there are exceptions.
What is a half-full electron configuration for Copper Cu. The electron configuration for Gallium Ga is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 Gallium Ga has 31 protons and 31 electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information.
Solved Give the full electron configuration for sulfur. Sulfur S2- How many valence electrons does Magnesium Mg have. The p orbital can hold up to six electrons.
_13Al 1s22s22p63s23p1 A shorthand way of writing this is to use the preceding noble gas configuration by putting its symbol in square brackets in front of the valence electrons. This is the best answer based on feedback and ratings. 1s22s22p63s23p4 But sulfur has 6 valence electrons tends to have a 2- charge and forms 2 bonds.
Sulfur is an electronegative element. The number of unpaired electrons in the last orbit of an element is the valency of that element. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital.
Its electron configuration is. The next six electrons will go in the 2p orbital. Calculate the relative atomic mass of the nickel in the sample.
The full electron configuration for sulfur atom is 1s22s22p63s23p4. For this the valency valence of sulfur is 4. And the 3s2 3p4 isnt the right answer.
SulfurS is the 16th element in the periodic table and its symbol is S. The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4. Sulfurs atomic number is 16.
The s sub level two electrons can occupy it. The electron configuration for sulfur is. The number of electrons is equal to the atomic number.
The full electron configuration for sulfur atom.
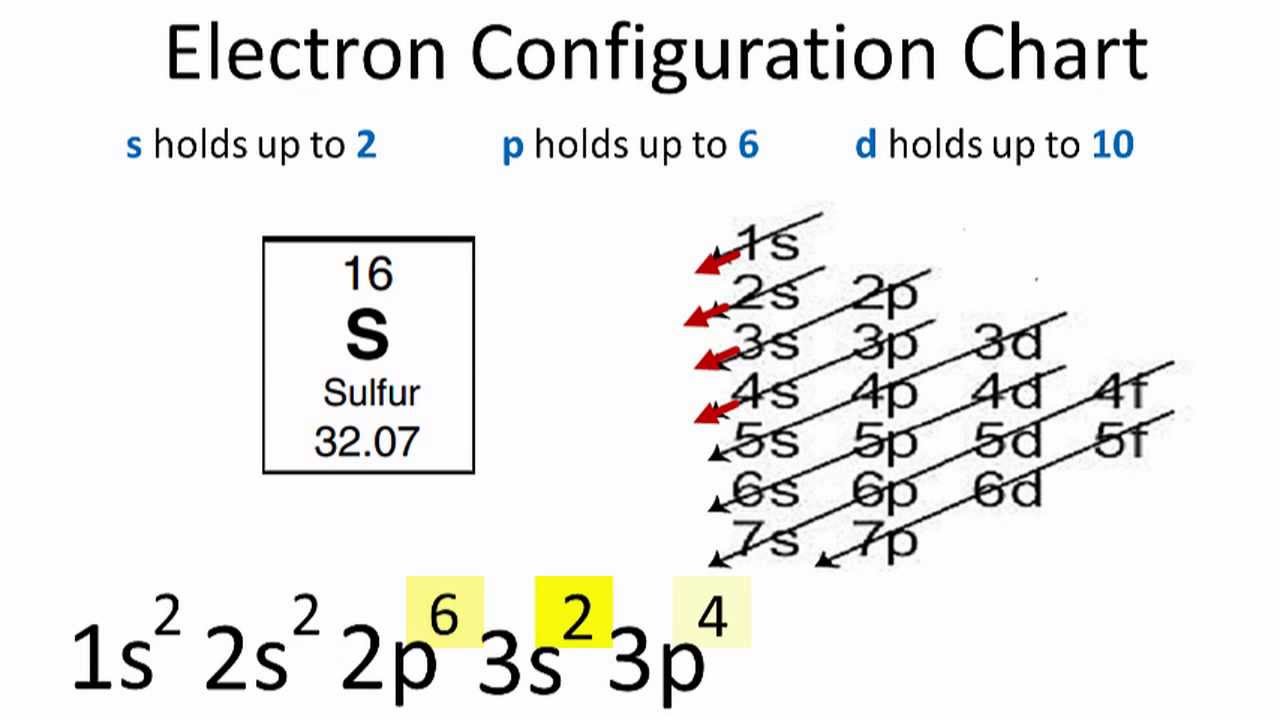
Sulfur Electron Configuration Youtube
![]()
Sulfur Electron Configuration What S Insight

Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Youtube
No comments for "Give the Full Electron Configuration for Sulfur."
Post a Comment